While vast information exists at the cellular level, understanding life’s processes remains incomplete due to the complex interaction of mechanical, diffusion, convective, biochemical, and cellular biology factors. To tackle these challenges, our research combines efforts from medical practitioners, in-vivo experimentalists, and computational scientists to develop models that bridge biological and physical scales.
Our research focuses on improving computational models of transport-reaction and mechanical systems to address biomedical challenges. Through close interdisciplinary collaboration, our work aims to develop advanced biophysical models that can inspire new therapeutic innovations.
Effects of Blood Transfusion on Oxygen Delivery
Blood transfusion is widely used to restore/increase in oxygen (O2) carrying capacity. We develop a mathematical model of the consequences of treating anemia by introducing red blood cells (RBCs) into the circulation via blood transfusion. Such an analysis serves to identify variables that may be manipulated to increase the rate of oxygen delivery (DO2) to the tissues, independently of changing blood O2 carrying capacity.
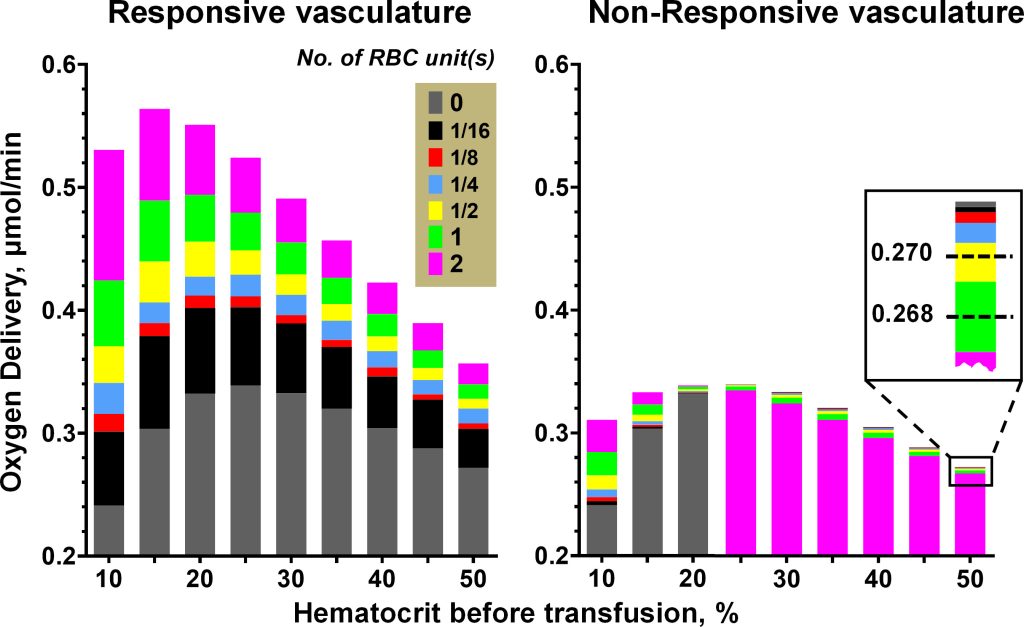
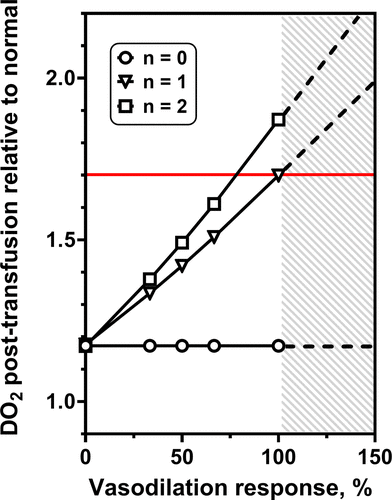
Our predictive model of DO2 in anemia describes the interaction between the mechanistic effects and vascular responses. Our modeling study strives to elucidate how the treatment of normovolemic anemia with blood transfusion impacts DO2 by the microcirculation. Coupled with experiments in hamsters, our model suggests a strategy for sustainable blood management to solve global blood shortage.
Rapid Treatment of Acute Respiratory Distress Syndrome
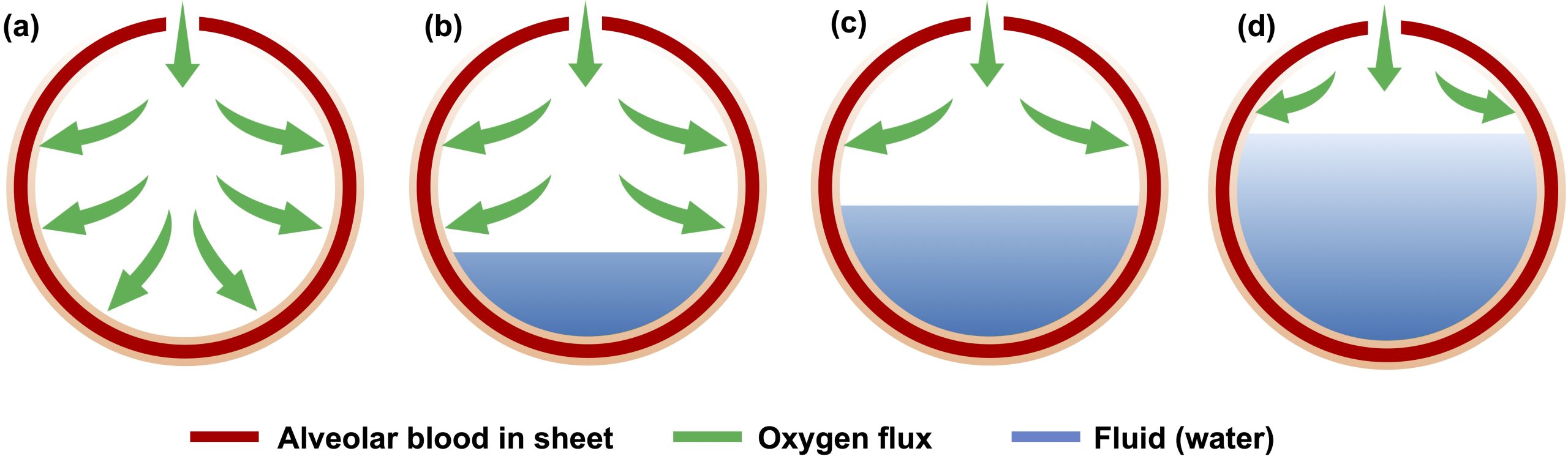
The COVID-19 infection is associated with the hindrance of blood oxygenation due to fluid accumulation in the lung alveoli. This is due to cytokines activating immune cells to eliminate SARS-CoV-2 infected cells, causing the accretion of fluid and cellular debris in the lungs, a condition similar to the Acute Respiratory Distress Syndrome (A.R.D.S.). We analyze how osmotic effects can be leveraged to extract fluid from the lungs by increasing blood NaCl concentration and the blood plasma osmotic pressure. Our mathematical model calculates the change of blood oxygenation with time, as O2 diffusion hindrance decreases upon absorption of the alveolar fluid into the pulmonary circulation. The predictions of our model point to hypertonic perfusion as a potential rapid treatment of A.R.D.S. caused, e.g., by COVID-19 infection, in tens of minutes.
Selected Publications
- Li et al., “Hypertonic treatment of acute respiratory distress syndrome.”, Frontiers in Bioengineering and Biotechnology, 11, doi: 10.3389/fbioe.2023.1250312, 1250312, 2023. PDF
- Li et al., “A model of anemic tissue perfusion following blood transfusion shows critical role of endothelial response to shear stress stimuli.”, Journal of Applied Physiology, 131(16), doi:10.1152/japplphysiol.00524.2021, 1815-1823, 2021. PDF